
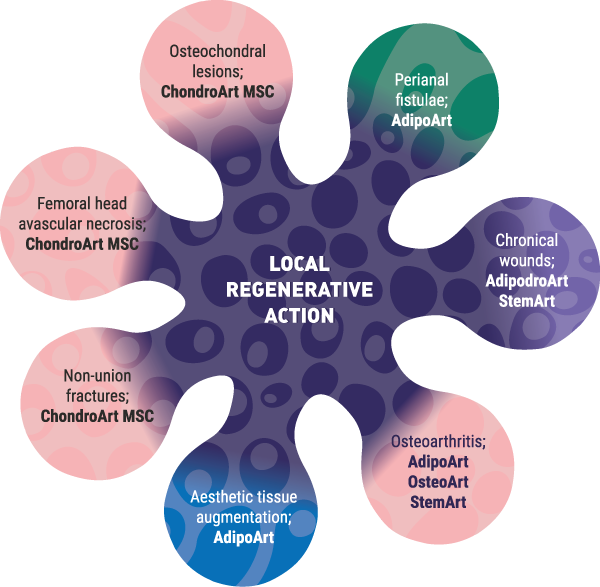
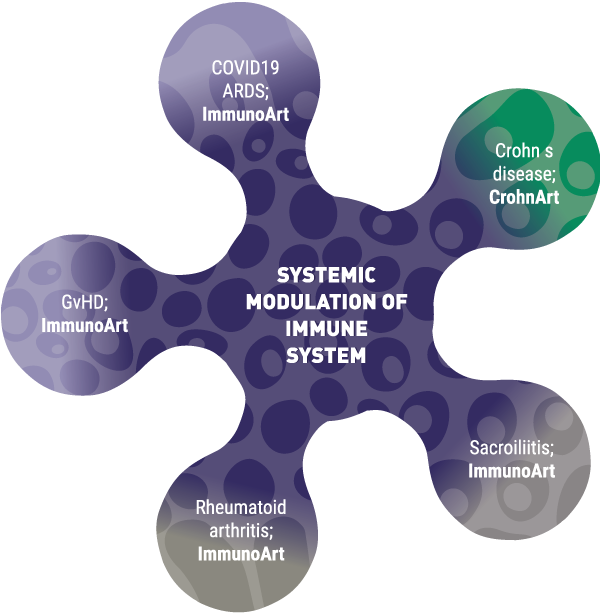
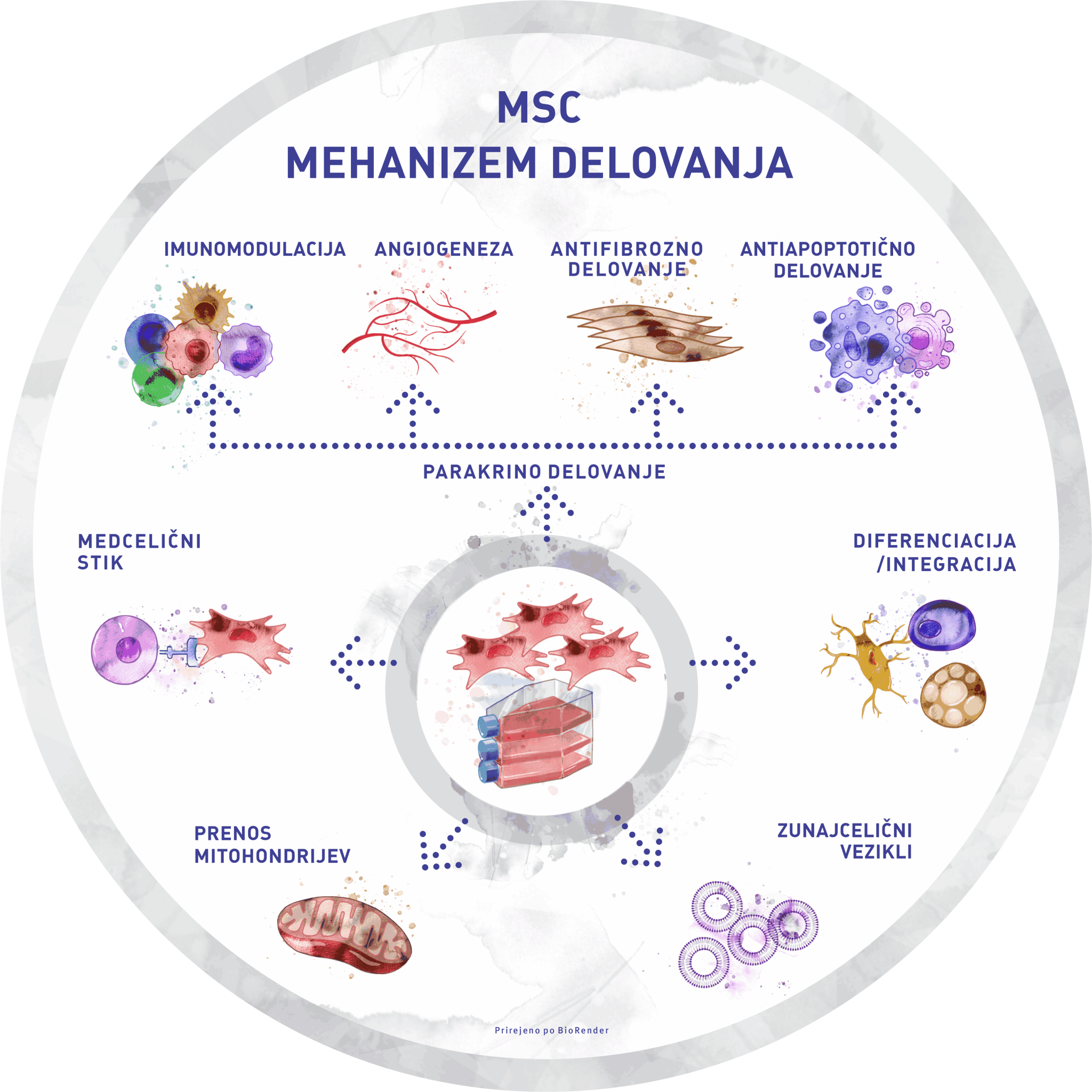
In 2008, GaiaCell was the first cell and tissue establishment in Slovenia to receive this status and has maintained the accreditation to date. In addition to tissue processing for ATMP production, the certificate also allows us to import and distribute human allografts. We are, therefore, a regional centre for human allograft tissue procurement for orthopaedics, traumatology, wound healing and others. Our long-lasting partnership with different tissue banks from the EU and USA enables us to ensure the stable and constant procurement of tissues needed by our partner surgeons.
Allografts are donated human tissues that are most commonly used in tissue reconstruction.
We source allografts from renowned tissue banks in Europe and America: